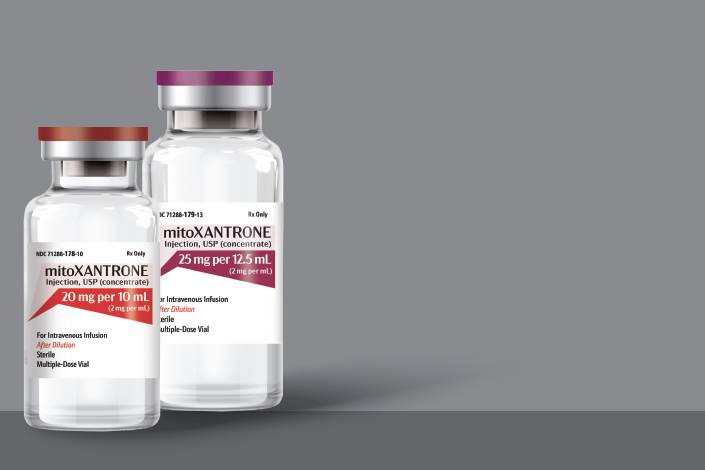
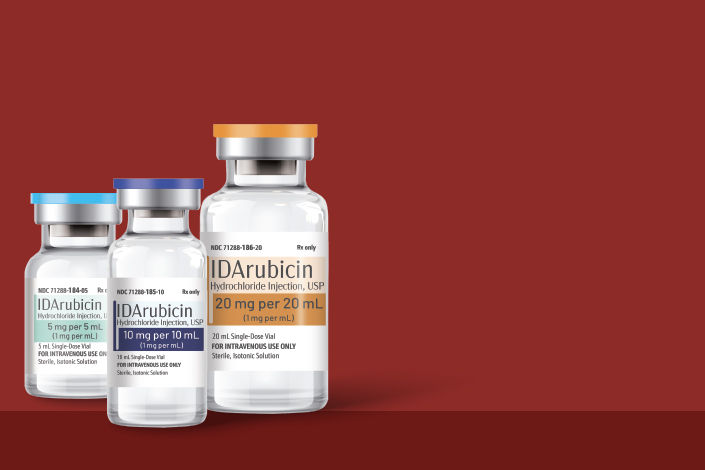
Meitheal Pharmaceuticals (Meitheal) is pleased to announce the official launch of DAUNOrubicin Hydrochloride Injection:
- 71288-182-05 – DAUNOrubicin Hydrochloride Injection 20 mg per 4 mL (5 mg per mL) SDV
ABOUT DAUNOrubicin HYDROCHLORIDE INJECTION
Daunorubicin hydrochloride injection in combination with other approved anticancer drugs is indicated for remission induction in acute nonlymphocytic leukemia (myelogenous, monocytic, erythroid) of adults and for remission induction in acute lymphocytic leukemia of children and adults.
For full prescribing information, please click on the following link.
Learn more about who we are and what we do at: www.meithealpharma.com.